Glycative stress and Anti-aging
Glycative stress biomarker measurement method (2)AGEs measurement
Glycative stress biomarker measurement method (2)
AGEs measurement
Glycative Stress Research Center, Faculty of Life and Medical Sciences, Doshisha University
Supervised by Professor Masayuki Yagi
When it comes to glycative stress evaluations, various substances that are generated in the process of non-enzymatic reactions of glucose and proteins in the body, can be a biomarker. Here we would like to explain about the AGEs measurement method and the non-invasive measurement method for AGEs in the body.
AGEs measurement
AGEs that are produced in blood via the late stage reactions include various substances such as Nε-carboxymethyl lysine (CML)1), pentosidine2) and Nω-carboxymethylarginine (CMA)3).
CML is a non-fluorescent/non-cross-linkable AGE, which is produced from lysine via GO (glyoxal) as the intermediate and is produced when diabetes and oxidative stress advance as well. Adding collagen that became CML to the medium in human dermal (skin) fibroblasts induces apotosis 4). It also exists on the epidermal layer whose metabolic turnover is relatively fast 5). CML accumulation in the stratum corneum has led to skin texture loss 6).
Pentosidine is a fluorescent/cross-linkable AGE, which is efficiently produced from ribose, arginine and lysine, and is covered by insurance as one of the early clinical biomarkers for nephropathy. In recent years, Pentosidine in blood and urine is gathering attention as a biomarker that reflects the aging of bone quality and is expected to be used for diagnosing osteoporosis 7). Furthermore, collagen in dermis also includes pentosidine whose amount increases with aging. When comparing diabetics to healthy people who are the same age, the amount of pentosidine accumulated in the dermis tends to be larger for diabetics 8).
CMA is one of the AGEs, which is produced from arginine via GO as the intermediate, and specifically remains in place in collagen 3).
For measuring CML in blood and pentosidine 9-10), ELISA kits which are sold by many companies are available for use. However, in order to measure AGEs in biological samples, treatment that matches the characteristics of the glycative reaction product to be measured is required. If a blood sample is heat-treated at a high temperature during CML or pentosidine measurement, AGEs are artificially generated during sample pretreatment, which may cause an error due to higher measurement values 11).
The HPLC method can be used as a countermeasure to avoid the deviation towards higher values at the time of pentosidine measurement. In the HPLC method, a serum sample is hydrolyzed with hydrochloric acid and contaminants in the sample are removed via pretreatment using an ion-exchange column, etc. Then, pentosidine is separated and analyzed via reversed-phase HPLC (Fig. 1)12). To detect pentosidine, pentosidine’s specific fluorescence (excitation wavelength: 335 nm, fluorescence wavelength: 385 nm) is used. However, the HPLC method has some issues such as a decrease in recovery rate during pretreatment, avoidance of contamination of measurement peaks with contaminants, and difficulty measuring multiple samples.
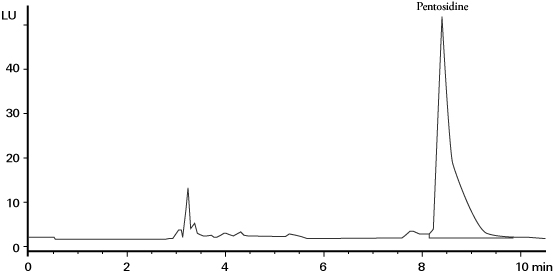
Fig. 1 Serum pentosidine measurement chromatograph via the HPLC method 12)
Column: Vydac 218TP54 (250 x 4.6 mm ID, 5 µm)
Eluent: 0.1% HFBA/acetonitrile (82.5/17.5), Flow rate: 1.0 mL/min,
Temperature: 30°C, Detection: fluorescence (ex 330 nm/em 375 nm)
Non-invasive measurement of AGEs pertaining to the skin (dermis) via AGE Reader®
AGEs that the skin accumulates is thought to be a factor that causes skin aging due to decrease in skin firmness, elasticity, etc. The amount of fluorescent AGEs that the skin accumulates can be non-invasively measured as the fluorescence intensity (auto fluorescence; AF) of the skin via the AGE Reader (Diagnoptics Technologies B.V.) 13-14).
The AGE Reader is a clinical testing device that was researched and developed for the purpose of evaluating the risk of developing diabetes complications by non-invasively measuring the amount of AGEs that the skin accumulates 15-17). This device measures AGEs by taking advantage of the characteristic that the fluorescent AGEs that have accumulated in the tissues are excited to emit a unique autofluorescence when irradiating the skin with ultraviolet rays (Fig. 2).
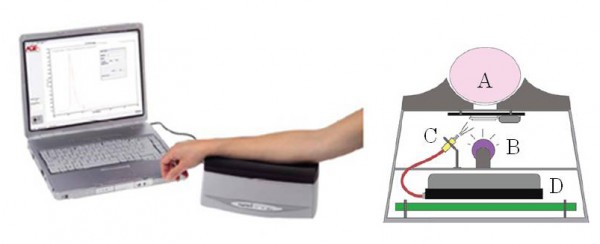
Fig. 2 Pattern diagram of AGE Reader ® measurement system and the main body configuration
A: Arm (measurement position), B: Light source (Black light),
C: Glass fiber, D: Spectroscope
In the AGE Reader measurement system, the light source (UV-A black-light) installed in the box-shaped main body (280 mm (length) × 150 mm (width) × 115 mm (height)) emits a 345 to 410 nm (maximum wavelength: 365 nm) wavelength of light that irradiates the skin surface through a glass window (approximately 2 × 2 cm) as excitation light. This device analyzes the obtained information using the dedicated software by introducing 420 to 600 nm of fluorescence emitted from skin through the excitation light into the spectroscope via the glass fiber, and then AF is calculated 18). The measurement time is about 90 seconds (in 3-measurement mode). The AGE Reader also measures the skin reflected light (reflection; Ref) via the white LED light at the same time as the fluorescence. If the Ref is less than 12%, the measured value is corrected. However, measurement cannot be performed when Ref is less than 6%. Therefore, subjects with darker skin (Fitzpatrick class 5-6 skin color) cannot be measured. Also, since the measurement values are affected by the use of skin care cosmetics including sunscreen, it is necessary to thoroughly wash the measurement position beforehand.
Skin AF is calculated from the ratio of fluorescence (420 to 600 nm) intensity to excitation light (300 to 420 nm) and is displayed in arbitrary units (AU). The AF calculated by the AGE Reader is output along with the comparison graph of data accumulated by the manufacturer. (subject’s sex, age, etc.) However, the AF analysis formula has not been disclosed by the manufacturer.
The AF values measured via the AGE Reader have shown the relationships between aging in diabetics and healthy people with fair skin (Fitzpatrick class 1-3 skin color). It shows that the values deviate upwards due to aging and diabetes.
The skin AGEs measurement position recommended by the manufacturer is the forearm. While there is less of a burden on a subject via the forearm measurement, it is greatly affected by the UV exposure depending on the season. The results of comparing measurement positions with fair-skinned people shows that the forearm and lower limbs are strongly correlated with diabetes complications 19).
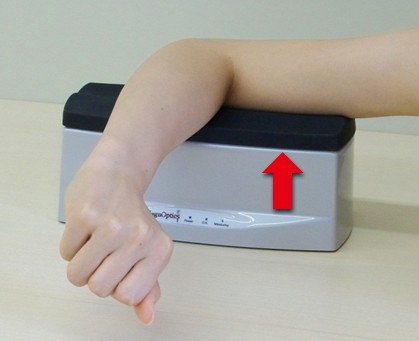
On the other hand, Japanese skin becomes red when exposed to ultraviolet rays and the skin color becomes darker. So, if Japanese people are subjected to the measurements, they are not easily affected by ultraviolet rays, making stable measurement possible at a position recommended for skin measurement tests: approximately 10 cm away from the tip of the right elbow on the inner part of the upper arm (Fig. 3)20-21). Skin AGEs measurement position via the AGE Reader needs to be selected appropriately according to the influence of ultraviolet rays, skin color and the race of subjects to be measured.
Fig. 3 Measurement of the inner part of the upper arm via AGE Reader ®
The red arrow indicates the measurement position.
Stratum corneum CML measurement via the tape stripping method
The stratum corneum is positioned at the outermost layer of the skin and is composed of stratum corneum cells and intercellular substances. The stratum corneum is constantly reconstituted by the stratum corneum cells that are successively produced from the epidermal layer and those that peel off gradually from the surface. The biological defense functions such as water retention and barrier property retention are maintained thanks to the stratum corneum. Changes in skin due to aging are often studied histologically and there are only a few simple biochemical measurement methods.
As a method for non-invasively evaluating the stratum corneum functions, there is the tape-stripping method in which stratum corneum cells are collected using an adhesive tape. In the tape-stripping method, some morphological evaluation methods have been reported pertaining to the number of exfoliated keratinocytes, keratinocyte area, cell morphology and nuclear residual rate.
Biochemical measurements of proteins, keratin 22), cathepsin 23), transglutaminase 24), arginase 25), etc. have been reported as well, based on the stratum corneum that was collected via the tape-stripping method.
The measurement of CML in the stratum corneum via the tape-stripping method makes it possible to simply collect and directly measure the state of glycative stress pertaining to proteins contained in the skin 26). Stratum corneum can be collected from a subject by pressing a commercially available adhesive sheet such as keratin checker AST-01 (manufactured by Asahi Biomed) against the inner part of the right upper arm for about 5 seconds with your fingers and then remove it from your skin (Fig. 4). After cutting the adhesive sheet that collected the stratum corneum and then homogenizing it via a Tris-HCl buffer solution (pH 7.5) using a micro homogenizer, etc. to scrape off the adhesive on the surface of the adhesive sheet, the stratum corneum extract is collected. To measure CML in the stratum corneum extract, a commercially available measurement kit that uses the ELISA method is used. At the same time, the amount of protein in the stratum corneum protein extract is separately measured and then the amount of CML per stratum corneum protein is calculated.
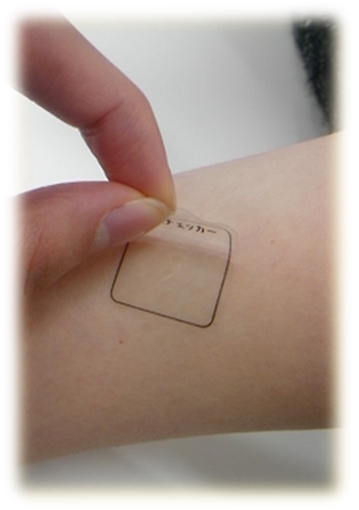
Fig. 4 Stratum corneum tape-stripping method
Inner part of the upper arm
References
-
- Nagai R, et al. : Biochem Biophys Res Commun. 1997; 234: 167-172
- Sell DR, et al. : J Biol Chem. 1989; 264: 21597-21602
- Iijima K, et al. : Biochem J. 2000; 347: 23-27.
- Alikhani Z, et al. : J Biol Chem. 2005; 280: 12087-12095.
- Kawabata K, et al. : Biochimica et Biophysica Acta. 2011; 1814: 1246?1252.
- 五味貴優 : BIO INDUSTRY. 2011; 28: 20-26.
- Saito M, et al. : Osteoporos Int. 2006; 17: 986-995.
- Dyer DG, et al. : J Clin Invest. 1993; 91: 2463-2469.
- Tahara N, et al. : Cardiovascular Therapeutics. 2012; 30: 42?48.
- Sanaka T, et al. : Nephron. 2002; 91: 64-73.
- Nakano M, et al. : Anti-Aging Medicine. 2010; 7: 92-93.
- Slowik-Zylka D, et al. : J Biochem Biophys Methods. 2004; 61: 313-329.
- Meerwaldt R, et al. : Diabetologia. 2004; 47: 1324-1330.
- Meerwaldt R, et al. : J Am Soc Nephrol. 2005; 16: 3687-3693.
- Meerwaldt R, et al. : J Am Soc Nephrol. 2005; 16: 3687-3693.
- Lutgers HL, et al. : Diabetes Care. 2006; 29: 2654-2659.
- Nomoto K, et al. : Anti-Aging Medicine. 2010; 7: 73-84.
- AGE Reader User manual Ver2.3, DiagnOptics, pp29.
- Tanaka K, et al. : Ther Apher Dial. 2010; 14: 334-340.
- Nomoto K, et al. : Anti-Aging Medicine. 2012; 9: 165-173.
- Miyazaki R et al. : Anti-Aging Medicine. 2009; 6: 83-94.
- 公開特許公報(A), 特開2008-249429.
- 田原祐助ら:BUNSEKI KAGAKU. 2009; 58: 15-19.
- 公開特許公報(A), 特開2001-29099.
- 公開特許公報(A), 特開2010-151482.
- Kamitani Y, et al. : Anti-Aging Medicine. 2013; 10: 55-59.
Glycative stress and Anti-aging
- What is glycative stress?
- Glycative stress biomarker measurement method (1) Measurement of blood glucose, glycated protein and glycation reaction intermediate
- Glycative stress biomarker measurement method (2) AGEs measurement
- Glycative stress biomarker measurement method (3) Evaluation of anti-glycative effects
- Glycative Stress and AGEs Receptors
- What is kidney disease?
- Glycative Stress and Skin Aging
- Glycative stress and arteriosclerotic disease
- Glycative stress and schizophrenia
- Glycative stress and liver disease
- Glycative stress and infertility
- Glycative stress and Alzheimer’s disease
- Glycative stress countermeasures (1) Blood glucose control
- Glycative stress countermeasures (2) Inhibition of glycation reaction
- Measures against glycative stress (3) Degradation and excretion of AGEs
- Measures against glycative stress (4) AGEs contained in food
- Issues and prospects of glycative stress countermeasures