Glycative stress and Anti-aging
Measures against glycative stress (3) Degradation and excretion of AGEs
Measures against glycative stress (3) Degradation and excretion of AGEs
Excretion of AGEs by the kidneys
AGEs released in the blood due to protein metabolism and AGEs absorbed from the digestive tract following the digestion of food are excreted into the urine via the kidneys. The proximal tubule of the kidney has a membrane receptor called megalin, which reabsorbs low-molecular-weight proteins that are filtered from urine. After AGEs in the blood bind to the megalin in the kidneys, they are taken into the renal tubular cells by endocytosis, an action where cells take in extracellular substances (Fig. 1) 1). However, when large amounts of AGEs are taken in by megalin, the degradation of AGEs in lysosome, which is one of the intracellular organelles, is saturated, resulting in the accumulation of AGEs in the renal tubular cells 2-3).This leads to reduced renal functions and an increase in the accumulation of in vivo AGEs.
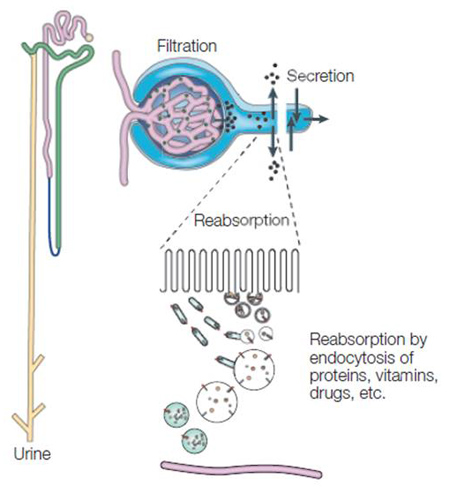
Fig. 1 Protein metabolism and excretion in the kidney (Adapted from Christensen et al .1))
Modification and metabolism of protein due to glycative/oxidative stress
Protein alteration plays a key role in the decline of functions in the nervous, immune and endocrine systems, as well as various cell and tissue functions associated with aging. Glycative stress and oxidative stress promote in vitro chemical modification of proteins, which then causes an abnormality. These proteins, which are called altered proteins, not only have reduced in vivo functions but also affect the cells and tissues 4). β-Amyloid, which accumulates in the brains of patients with Alzheimer’s disease, is a type of altered protein that damages nerve cells and impairs neural functions such as memory and learning functions. Also, crystalline in the lens of the eye is denatured along with aging, and its aggregation (protein alteration) causes turbid to become a cause of cataracts. Furthermore, many neurodegenerative diseases such as Parkinson’s disease are caused by the accumulation of altered proteins that have changed structures 5).
Normally, altered proteins are degraded by a proteolytic enzyme called proteasome. There are 2 types of proteasomes: 26S (molecular weight: 2.5 million) and 20S (molecular weight: 700,000). 26S proteasomes degrade many altered proteins after the altered proteins have undergone ubiquitination. Also, oxidized proteins are degraded by 20S proteasomes (Fig. 2) 5). On the other hand, AGEs derived from proteins are hardened and denatured due to the formation of cross-linking proteins, which makes them less susceptible to degradation with protease 6).
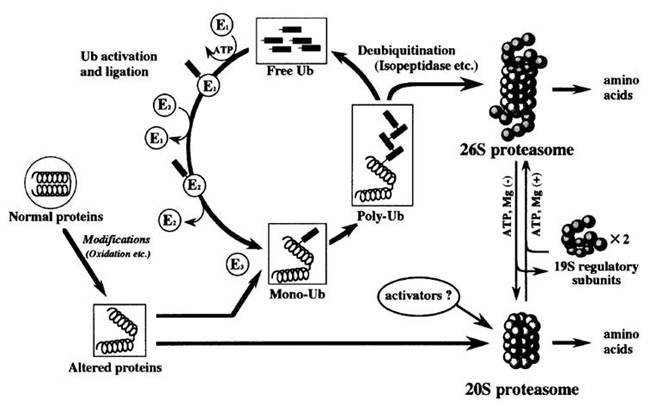
Fig. 2.Degradation action of ubiquitinated protein by proteasome (Goto S, et al.(2001) 4))
The body always removes altered proteins that are produced inside the cells by repeating synthesis and degradation of proteins and reuses the amino acids produced by the degradation to maintain its functions. However, the protein synthesis ability of cells decreases with aging, and metabolic turnover slows down in older animals. Furthermore, degradation of cellular protein becomes difficult due to the formation of AGEs which comes with aging, resulting in an increased amount of in vitro altered proteins.
Substances with degradation effects on AGE-crosslinks
N-phenacylthiazolium bromide (PTB) is known as a substance with degradation action on AGE-derived crosslinks of proteins 7). PTB recognizes the α-diketone structure of Amadori-protein-ene-dion-derived protein, which is formed by glycative stress, and degrades the C-C bonds and protein-protein crosslinks (Fig. 3) 7). It is suggested that this action may contribute to the inhibition of AGE accumulation in blood vessels and the treatment of vascular complications that come from diabetes. A study where 10 mg/kg of PTB was orally administered to diabetic rats for 4 weeks indicated that it inhibits the formation of collagen AGE-derived crosslinks 7) and acted to degrade AGEs in the blood vessels 8). Furthermore, inhibition actions on vascular stiffening and accumulation of AGEs were observed in a conducted study where 3-phenacyl-4,5-dimethylthiazorium chloride (ALT-711) with increased PTB water solubility was administered to diabetic rats 9). A human clinical study conducted on the oral ingestion of ALT-711 for 8 weeks at amounts of 210 mg/day or 420 mg/day, demonstrated an improvement in vascular stiffening and uncontrolled systolic blood pressure 10-11). PTB is referred to as an “AGE breaker” because of these results.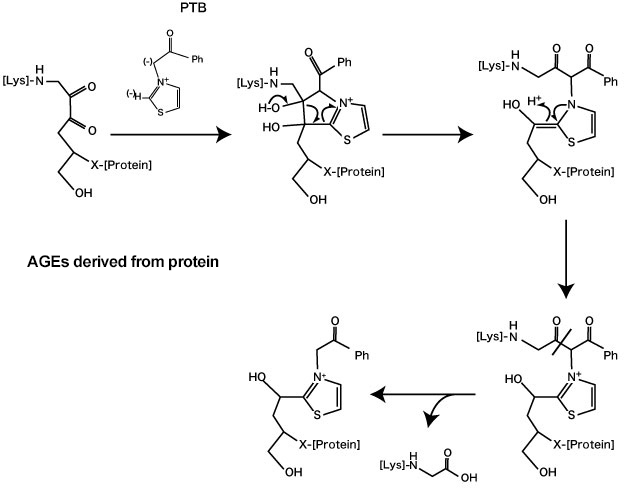
Fig. 3. Degradation action of AGE-derived crosslinks by N-phenacylthiazolium bromide (PTB)
(Vasan S, et al. (1996) 7)
On the other hand, another study reported that PTB did not affect the degradation of AGE-derived crosslinks in the skin and tail collagen of diabetic rats 12). For this reason, there is some skepticism about the effects of PTB.
Plant ingredients that are known for having the same effect as PTB include extracts of Japanese mugwort (Artemisia indica), rooibos (Aspalathus linearis), Chinese milk-vetch (Astragalus sinicus) 13), yuzu (Citrus junos) 14) and pomegranate (Punica granatum) 15).
YAC extract, which is ethanol extract from Japanese mugwort (yomogi), degraded AGE-crosslinks generated in the collagen gel-glucose reaction model. Furthermore, a study conducted on the usage (6 months) of skin lotion, milky lotion and cream containing the YAC extract, showed improvement in the elasticity and yellowness (b*) of skin 13).
Terpinen-4-ol, a kind of monoterpene alcohol contained in yuzu (Citrus junos) has been shown to affect degradation action on AGE-derived crosslinks through the hydrolysis of acid anhydride via the production of carboxylate ester by the reaction type of Bayer-Villiger oxidation after nucleophilic substitution by hydroperoxide 14).
It is presumed that the trihydroxybenzene structure of ellagitannins is involved in the degradation action of pomegranate extract and pomegranate-derived ingredients on AGE-derived crosslinks 15).
The degradation action on AGE-derived crosslinks by these natural products may contribute to the degradation and excretion of in vivo AGEs that have already accumulated.
Degradation action on AGEs by oxidative proteolytic enzymes 13)
Oxidized protein hydrolase (OPH) is a kind of serine protease that is widely present in living tissues such as porcine liver, human blood and rat brain 16-17). It has also been reported that OPH is present in the stratum corneum of human skin 18-19). It is also known as an acylamino-acid releasing enzyme (AARE) since it is an enzyme that releases N-terminal acylated amino acids into proteins. OPH acts on the degradation of N-terminal amino acids in formylated, acetylated, butylated and propylated proteins in addition to acylated proteins 20) as well as aging proteins that have been modified by oxidation and glycation 21). Furthermore, OPH and proteasome act together on the degradation of aging proteins 22). When it comes to diabetic rats, serum OPH activity significantly increases and the amount of carbonyl-modified protein in the blood decreases 23).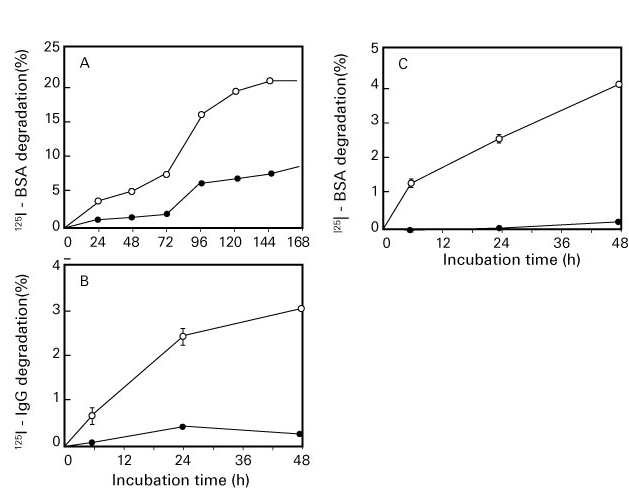
Fig. 4. Degradation action on oxidized/glycated proteins by oxidized protein hydrolase (OPH)
(Fujino T, et al .21))
A: oxidized bovine serum albumin (BSA), B: oxidized immunoglobulin G (IgG ), C: glycated BSA
●: Untreated (control), ○: Oxidized or glycated protein
Natural products that are known to affect OPH activity are plant extracts such as tea, herbs and vegetables. There are some herbs and herbal teas that promote OPH activity. On the other hand, teas derived from tea trees (Camellia sinensis) and catechins inhibit OPH activity 24-26). OPH has a degradation action on glycated proteins and AGEs, which are widely present in living tissues. This means that the activation of OPH activity by natural products may contribute to the promotion of degradation and excretion of in vivo AGEs.
References
-
- Christensen E, et al.:Nat Rev Mol Cell Biol. 2002; 3: 256-266.
- Miyata T, et al.:Kidny Int, 1998 ; 53 : 416-422.
- Gugliucci A, et al.:Diabetologia, 1996 ; 39 : 149-160.
- Goto S, et al.:Ann N Y Acad Sci. 2001; 928: 54-64.
- Carrard G, et al.:Int J Biochem Cell Biol. 2002; 34: 1461-1474.
- Schnider, et al.:J Clin Invest. 1981; 67: 1630-1635.
- Vasan S, et al.:Nature. 1996; 382: 275-278.
- Cooper ME, et al.:Diabetologia. 2000; 43: 660-664.
- Freidja ML, et al.:Cardiovascular Diabetology 2014; 13: 55-71.
- Bakris GL, et al.:Am J Hypertens. 2004; 17: 23S-30S.
- Kass DA, et al.:Circulation. 2001; 104: 1464-1470.
- Yang S, et al.:Arch Biochem Biophys. 2003; 412: 42-46.
- 多田明弘:COSMETIC STAGE. 2011; 5: 33-38.
- Nagamatsu R, et al.:Biosci Biotechnol Biochem. 2012; 76: 1904-1908.
- Yagi M, et al.:Glycative Stress Research. 2015; 2: 58-66.
- Mitta M, et al.:J Biochem. 1998; 123: 924-931.
- Fujino T, et al.:J Biochem. 2000; 127: 1081-1086.
- 石神政道ら:第14回日本抗加齢医学会総会プログラム抄録集. 2014: 349.
- 石神政道ら:第15回日本抗加齢医学会総会プログラム抄録集. 2015: 239.
- Krishna RG, et al.:Protein Science. 1992; I: 582-589.
- Fijino T, et al.:J Biochem. 1998; 124: 1077-1085.
- Shimizu K, et al.:Biochemi Biophys Res Commun. 2004; 324: 140–146.
- Shimizu K, et al.:Biol Pharm Bull. 2009; 32: 1632-1635.
- 八木雅之ら:第13回日本抗加齢医学会総会プログラム抄録集. 2013: 253.
- 三橋璃子ら:第13回日本抗加齢医学会総会プログラム抄録集. 2013: 254.
- 三橋璃子ら:第14回日本抗加齢医学会総会プログラム抄録集. 2014: 362.
Glycative stress and Anti-aging
- What is glycative stress?
- Glycative stress biomarker measurement method (1) Measurement of blood glucose, glycated protein and glycation reaction intermediate
- Glycative stress biomarker measurement method (2)AGEs measurement
- Glycative stress biomarker measurement method (3) Evaluation of anti-glycative effects
- Glycative Stress and AGEs Receptors
- What is kidney disease?
- Glycative Stress and Skin Aging
- Glycative stress and arteriosclerotic disease
- Glycative stress and schizophrenia
- Glycative stress and liver disease
- Glycative stress and infertility
- Glycative stress and Alzheimer’s disease
- Glycative stress countermeasures (1) Blood glucose control
- Glycative stress countermeasures (2) Inhibition of glycation reaction
- Measures against glycative stress (3) Degradation and excretion of AGEs
- Measures against glycative stress (4) AGEs contained in food
- Issues and prospects of glycative stress countermeasures