Glycative stress and Anti-aging
Glycative stress countermeasures (2) Inhibition of glycation reaction
Glycative stress countermeasures (2) Inhibition of glycation reaction
In vivo glycation reaction
Reducing sugars such as glucose, are predominantly involved in the in vivo non-enzymatic reactions with amino acids and/or proteins to form a Schiff base. Then, it undergoes an Amadori rearrangement to create Amadori product, a glycated protein, which is an irreversible substance.
Through the production of intermediates (mainly carbonyl products such as 3-deoxyglucosone (3DG), glyoxal, methylglyoxal, glyceraldehyde and glutaraldehyde), the Amadori products become advanced glycation end-products (AGEs).The glycation reaction, in a narrow sense, means a series of these reaction processes.
AGEs are formed through carbonylation that occurs between proteins, aldehydes and ketones, which are produced by alcohol metabolism and lipid oxidation in addition to glucose in the blood. Therefore, there are many kinds of AGEs substances with different production routes (Fig. 1) 1).
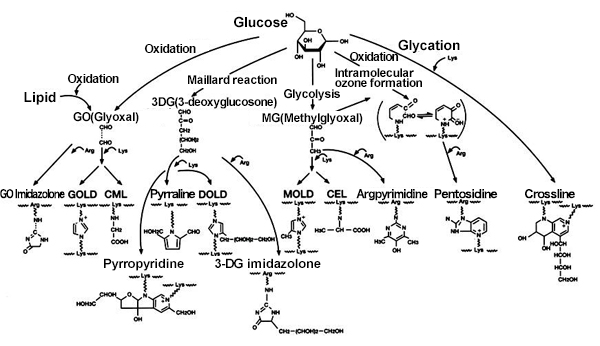
Fig. 1 In vivo production routes of AGEs
There are fluorescent and no-fluorescent substances in AGEs. Fluorescent AGEs include pentosidine, crossline, pyrropyridine, etc. Non-fluorescent AGEs include Nε–(carboxymethyl) lysine (CML), Nω-(carboxymethyl) arginine (CMA), etc. Also, since pentosidine, crossline, etc. possess protein cross-linking properties, AGEs derived from proteins lead to physical changes in tissues. AGEs that bind to the receptor RAGE (receptor for AGEs) will activate intracellular signals and induce the production of proinflammatory cytokines. For this reason, the in vivo production and accumulation of AGEs damages various cells and tissues.
The concept that comprehensively captures biological stress caused by reducing sugar or aldehyde loads and its subsequent reactions is called glycation stress. One of the measures against glycative stress is the inhibition of these in vivo glycation reactions.
In vitro inhibition effect on glycation reaction by natural products
The effects of inhibiting of glycation reaction is assessed by calculating IC50 (50% inhibitory concentration) of the test substance from the amount of glycation reaction intermediates and various AGEs produced in the reaction solution obtained by adding various test substances to the reaction systems of glucose and various proteins such as human serum albumin (HSA) and collagen 2).
Aminoguanidine, a glycation reaction inhibitor, is commonly used as a positive control for the inhibitory effects for glycation reaction. Inhibition of glycation reaction has been observed in many materials such as mixed herbs 3), edible purple chrysanthemum 4), Sasa senanensis Rehder 5), herbal tea 6), fruits 7), vegetables 8) using this evaluation system.
The inhibition of glycation reaction is presumed to be mainly attributed to polyphenols in plants. Substances that inhibit glycation reaction include phenolic acids (Fig. 2) such as cinnamic acid and benzoic acid analog, flavonoids (Fig. 3), isoflavones and procyanidins (Fig. 4) 9). Additionally, other phenolic compounds, terpenes, carotenoids, unsaturated fatty acids, polysaccharides, melanoidine and vitamins also have glycation reaction inhibition effects.
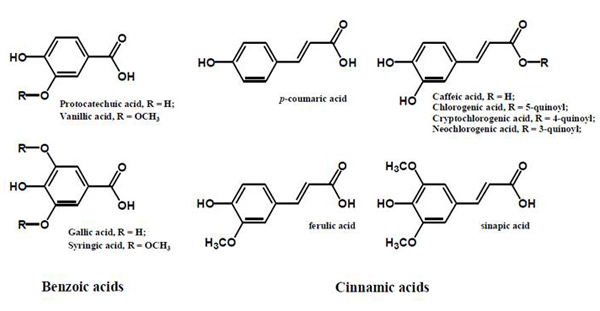
Fig. 2.Phenolic acids contained in plants 9)
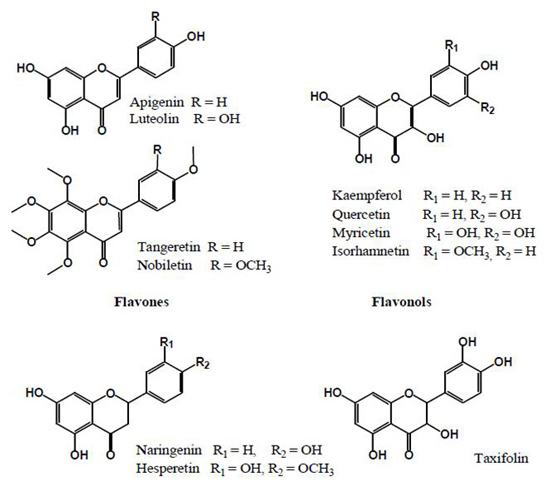
Fig. 3. Flavonoids contained in plants 9)
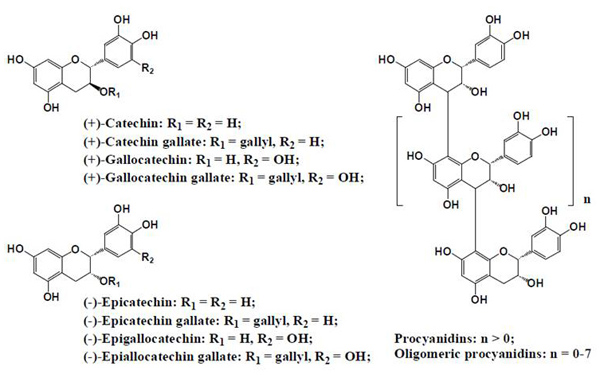
Fig. 4. Procyanidins contained in plants 9)
Many in vitro tests and animal tests have reported that some plant materials have anti-glycative effects. These effects primarily involve polyphenols. The distribution of polyphenols contained in plants is closely associated with evolution and classification and is one of the indicators for chemotaxonomy. Plants belonging to the same taxonomic family or tribe are presumed to contain polyphenols with a similar structure 10). On the other hand, the formation of in vivo AGEs has multiple routes with bypasses and branching, and furthermore, glycation and oxidation reactions are complexly intertwined (Fig. 1). For this reason, it is necessary to simultaneously inhibit multiple routes using multiple components to obtain a useful in vivo glycation reaction inhibition effects. It is essential to consider the taxonomic knowledge of plants when selecting and combining plant materials to achieve effective inhibition of glycative reaction.
In vitro inhibition effect on glycation reaction by mixed herb extracts
The mixed herb extract is one of the inhibitory materials for glycation reaction and is blended based on the findings that the hot water extract of the plants listed below demonstrate different inhibition effects on the formation of fluorescent AGEs, 3DG, pentosidine and CML. The plants used for a mixed herbal extract are the aerial part of Houttuynia cordata, fruit of Crataegus oxyacantha, flower of Chamaemelum nobile, leaf of Vitis vinifera, which are taxononically different plant species. They are familiar to Japanese people and have ample history as food. The mixed herb extract was commercialized as “AG Herb MIXTM” by ARKRAY, Inc. in 2006 as a functional food material 11). It is confirmed that one of the components that inhibits glycation reaction is chamaemeloside: apigenin 7-O-β-D-glucopyranoside-6’’-(3’’’-hydroxy-3’’’-methylglutarate) in Chamaemelum nobile 12).
The mixed herb extract inhibits the production of fluorescent AGEs, 3DG, and CML in an in vitro glycation reaction evaluation test system in a concentration-dependent manner. Compared to aminoguanidine, they have strong inhibition effects on the formation of CML and pentosidine. In addition, the mixed herb extract inhibits the production of fluorescent AGEs for collagen as well as HSA, and also inhibits the production of CMA that is specifically produced in collagen. It has been suggested that the mixed herb extract may inhibit multiple routes of glycation reaction while exhibiting an inhibitory effect on glycation reaction which is equal to or higher than that of aminoguanidine in the in vitro glycation reaction system, and it may effectively inhibit a complicated in vivo glycation reaction.
Effects of the mixed herb extract on streptozotocin-induced diabetic rats 13)
In this animal study, seven 6-week-old male Sprague-Dawley rats were divided into 4 groups using a completely random assignment design: control group (G1), streptozotocin (STZ) group (G2), mixed herb extract group (G3), and aminoguanidine group (G4). Each rat in the G2 to G4 groups was administered a single dose of streptozotocin (amounts varied per body weight: 55 mg/kg) in the tail vein to induce diabetes. They were held in captivity for 12 weeks. As feed, a MF powder diet for mice/rats (MF) was administered to the G1 and G2 groups , and a diet that had mixed herb extracts or aminoguanidine blended into it was administered to the G3 and G4 groups.
The results showed that both the mixed herb extract group (G3) and the aminoguanidine group (G4) showed a tendency of inhibition increases in serum pentosidine and CML compared to the STZ group (G2). A tendency to inhibit increases in wet kidney weight was also demonstrated. The mixed herb extract had a glycation reaction inhibition effect equal to or higher than that of aminoguanidine in STZ-induced diabetic rats, and was expected to prevent the progression of aging as well as reduce the risk of developing diabetic complications.
Study on the effects of mixed herb extract intake by pre-diabetic subjects 14)
In this human clinical study, 26 adult males and females with pre-diabetic conditions (casual blood glucose level of 110 to 199 mg/dL and HbA1c of 5.5 to 6.5% (JDS value)) were assigned to a test diet group and a placebo diet group (13 subjects respectively). They took either 3,000 mg/day of the mixed herb extract (5 times the recommended intake amount ) or the same amount of a placebo diet for 8 consecutive weeks. Blood was collected from each subject before and after the 8-week experimental diet to measure 3DG and CML as glycation reaction products in the blood. This clinical study was approved by the ethics committee and conducted according to the spirit of the Declaration of Helsinki. Subjects fully understood the content of the study, submitted a consent form, and participated voluntarily.
During the intake period, no adverse events (changes in health condition) that are presumed to be caused by the intake of the mixed herb extract were observed in the liver function, renal function, glucose/lipid metabolism function tests, etc., and the safety of taking the extract as a food was verified. Furthermore, the blood AGEs level before and after the 8 weeks of intake were measured and compared. Compared to the placebo diet group, the test diet group exhibited inhibition effects in the increase of blood 3DG (P = 0.094) in the analysis of the subgroup with blood glucose levels of 110 mg/dL or higher, and a significant decrease in CML (p = 0.048) in the analysis of the subgroup with HbA1c (JDS value) 5.9% or more. As a result, it was suggested that the mixed herb extract inhibits the formation of glycation reaction intermediates and AGEs in humans that are caused by a glycometabolism disorder.
Study on the effects of mixed herb extract intake by diabetic subjects 15)
In this human clinical study, 7 adult males and females with type 2 diabetes (HbA1c: 5.7 to 9.2% (JDS value)) took 600 mg/day of the mixed herb extract as a test diet for 12 consecutive weeks. Blood was collected from each subject before and after the 8 and 12 week marks of the test diet to measure 3DG and CML as glycation reaction products in the blood. Skin elasticity was measured with a Cutometer before and after the 2, 4, 8 and 12 weeks of intake. This clinical study was approved by the ethics committee and conducted according to the spirit of the Declaration of Helsinki. Subjects fully understood the content of the study, submitted a consent form, and participated voluntarily.
During the intake period, no adverse events (changes in health condition) that are presumed to be caused by the intake of the mixed herb extract were observed in the liver function, renal function, glucose/lipid metabolism function tests, etc., and the safety of taking the extract as a food was verified. In addition, 3DG was significantly reduced after 8 and 12 weeks (p < 0.001) and CML was significantly reduced after the 12 weeks (p <0.001) compared to before intake. These results suggest that the glycation reaction inhibition effects of the mixed herb extract, which have already been confirmed in vitro and in animal experiments, is also effective for humans. In addition, the skin elasticity significantly improved after 8 weeks (p = 0.001) and 12 weeks (p <0.001) compared to before intake (R2: Ua/Uf). It is thought that the intake of the mixed herb extract inhibited the formation of cross-linkable AGEs and resulted in inhibiting the hardening of proteins and improving the elasticity through skin turnover.
Effects of mixed herb extract on the inhibition of AGEs accumulation in skin 16)
In this human clinical study, a group of 8 healthy subjects and a group of 4 diabetic/hyperglycemic adult males and females took 600 mg/day of the mixed herb extract and a test diet containing ginger and zanthoxylum pipertium for 12 consecutive weeks. The accumulated amount of AGEs were measured with the AGE Reader before and after 8 and 12 weeks of intake. This clinical study was approved by the ethics committee and conducted according to the spirit of the Declaration of Helsinki. Subjects fully understood the content of the study, submitted a consent form, and participated voluntarily.
During the intake period, no adverse events (changes in health condition) that are presumed to be caused by the intake of the mixed herb extract were observed in the liver function, renal function, glucose/lipid metabolism function tests, etc., and the safety of taking the extract as a food was verified. Furthermore, the amount of AGEs accumulated in the skin significantly decreased after 8 weeks (p = 0.035) and 12 weeks (p = 0.020) in the healthy subject group when compared to before intake. A decrease was observed in 3 out of 4 patients in the diabetic/hyperglycemic group. Since fluorescent AGEs contain crossline and pentosidine that are involved in the formation of cross-linking proteins, it is suggested that the intake of the mixed herb extract may inhibit aging, such as hardening caused by the conversion of skin proteins into AGEs.
Effects of plant materials on inhibition of in vitro glycation reaction and inhibition of glycative stress in humans
The mixed herb extract demonstrated inhibition effects on glycative stress in humans via intake as well as inhibition effects on in vitro glycation reaction. These findings suggest that the mixed herb extract may have inhibited multiple routes of glycation reaction in the in vitro glycation reaction system and effectively inhibited complicated in vivo glycation reactions.
Results similar to the mixed herb extract have been obtained with single-material extract such as the following fruits: mangosteen (Garcinia mangostana) 17) and pomegranate (Punica granatum) 18). Mangosteen contains many types of tannins 19), phenolic acids 20) and xanthones 21), and pomegranate contains ellagitannins 22). Regarding the glycation reaction inhibitory effects brought by a single material, it is possible that these substances are decomposed into many kinds of low molecular weight substances that are easily absorbed intravitally by the intestinal flora and act on the multiple routes of the glycation reaction system 23).
The key to effectively utilizing the inhibition effects of glycation reaction, as a measure against glycative stress, will be the selection and combination of materials.
References
-
- 三浦雅一ら:生体内AGEsの測定, AGEs研究の最前線. 2004, 37-50, メディカルレビュー.
- Hori M, et al.:Anti-Aging Medicine. 2012 ; 9 : 125-134.
- Yonei Y, et al.:Anti-Aging Medicine. 2008 ; 5 : 93-98.
- Yagi M, et al.:Anti-Aging Medicine. 2012 ; 9 : 61-74.
- 堀未央ら:同志社大学理工学研究. 2011 ; 52 : 61-67.
- Hori M, et al.:Anti-Aging Medicine. 2012 ; 9 : 135-148.
- Lanny P, et al.:Anti-Aging Medicine. 2013 ; 10 : 70-76.
- Ishioka Y, et al.:Glycative Stress Research. 2015 ; 2 : 22-34.
- Odjakova M, et al.:Glycosylation. 2012 : 223-256, InTech
- Robards K, et al.:Analyst. 1997 ; 122 : 11R-34R.
- 八木雅之ら:COSMETIC STAGE. 2011 ; 15 : 23-32.
- 八木雅之ら:aromatopia. 2007 ; l16 : 26-29.
- Yonei Y, et al.:Anti-Aging Medicine. 2008 ; 5 : 93-98.
- Yonei Y, et al.:Anti-Aging Medicine. 2010 ; 7 : 26-35.
- Kubo M, et al.:J Clin Biochem Nutr. 2008 ; 43(Suppl 1) : 66-69.
- 田村隆朗ら:同志社大学理工学研究報告. 2012 ; 52 : 244-252.
- Ohno R, et al.:J Clin Biochem Nutr. 2015 ; 57 : 27-32.
- Yagi M, et al.:Glycative Stress Research. 2014; 1 : 60-67.
- Moosophin K, et al.:KKU Res J. 2010 ; 15 : 377-384.
- Zadernowski R, et al.:Food Chemistry. 2009 ; 112 : 685–689.
- Shan T, et al.:Curr Mol Med. 2011 ; 11 : 666–677.
- Ito H, et al.:Food Chem. 2014 ; 152 : 323-330.
- Verzelloni E, et al.:Mol Nutr Food Res. 2011 ; 55 : S35-S43.
Glycative stress and Anti-aging
- What is glycative stress?
- Glycative stress biomarker measurement method (1) Measurement of blood glucose, glycated protein and glycation reaction intermediate
- Glycative stress biomarker measurement method (2)AGEs measurement
- Glycative stress biomarker measurement method (3) Evaluation of anti-glycative effects
- Glycative Stress and AGEs Receptors
- What is kidney disease?
- Glycative Stress and Skin Aging
- Glycative stress and arteriosclerotic disease
- Glycative stress and schizophrenia
- Glycative stress and liver disease
- Glycative stress and infertility
- Glycative stress and Alzheimer’s disease
- Glycative stress countermeasures (1) Blood glucose control
- Glycative stress countermeasures (2) Inhibition of glycation reaction
- Measures against glycative stress (3) Degradation and excretion of AGEs
- Measures against glycative stress (4) AGEs contained in food
- Issues and prospects of glycative stress countermeasures