Glycative stress and Anti-aging
Glycative stress countermeasures (1) Blood glucose control
Glycative stress countermeasures (1) Blood glucose control
Hormonal regulation function of blood glucose and glycometabolism
The cells in vivo absorb glucose from blood and use it to synthesize ATP (adenosine triphosphate), an energy source. The brain largely depends on glucose when synthesizing ATP, which is why it is significantly affected by a drop in blood glucose levels. Therefore, in order to avoid a decrease in blood glucose levels and instead increase the level, there are various types of hormones such as glucagon, growth hormone, epinephrine or adrenaline, and corticosteroid in human body. These hormones help glucose enter skeletal muscles and adipocytes via glucose transporter, and control glycometabolism in cells in order to regulate the blood glucose levels. On the other hand, the only hormone that lowers the blood glucose level is insulin. This imbalance is thought to be a way to help humans survive hunger (Fig. 1) 1).
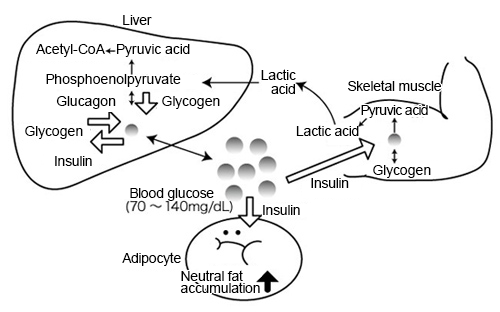
Fig. 1Adjustment function of glycometabolism and blood glucose
Ryoji Nagai, et al. (2013) 1)
As for healthy people, their fasting blood glucose levels fall within 70 to 90 mg/dL. However, postprandial blood glucose levels rise to around 140 mg/dL depending on dietary intake. The rising blood glucose levels are detected by the hypothalamus of the brain and the signal is transported to the β-cells (beta cells) of the pancreas via parasympathetic nerves. Then, insulin is secreted. GLUT2 (glucose transporter 2) is contained in β-cells as a glucose transporter, is dependent on the blood glucose levels (glucose concentration level in blood) and thus glucose is taken into the cells. Along with this, β-cells detect elevated blood glucose levels and secrete insulin. Insulin also promotes glucose uptake via GLUT4 (glucose transporter 4) in skeletal muscles and adipocytes, as well as acts on glycolysis to promote glycometabolism. As a result, the blood glucose level that became elevated via ingestion decreases to about 100 mg/dL.
When the hypothalamus detects a decrease in the blood glucose level, the sympathetic nerve stimulates α-cells (alpha cells) in the pancreas and glucagon is secreted. In the liver, glucagon is activated by phosphorylating glycogen phosphorylase, and the accumulated glycogen is decomposed and becomes glucose, which is to be secreted into the blood, causing the rise of blood glucose levels.
Changes in blood glucose levels due to meals are controlled mainly by the mechanisms of the above hormonal effects so that the level becomes about 100 mg/dL between meals.
Carbohydrate digestion/absorption and incretins
Polysaccharides (carbohydrates) contained in food ingredients are decomposed into disaccharides via α-amylase in saliva and pancreatic juice. Then, the disaccharides that have reached the small intestine are decomposed into monosaccharides via α-glucosidase in the brush border of mucosal epithelial cells which contain microvillus and then are absorbed. α-glucosidase includes maltase which decomposes maltose into two molecules of glucose, sucrase which decomposes sucrose into fructose and glucose, and isomaltase which decomposes isomaltose and converts it into two molecules of glucose1).
Two types of hormones, gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are secreted from the small intestine due to dietary ingestion stimulus in vivo. These gastrointestinal hormones are called incretins. Furthermore, when the foods are continuously ingested, the increased incretin activates pancreatic β-cells, which increases the level of insulin secretion and limits postprandial blood glucose elevation. Incretin is a gastrointestinal factor that prompts additional insulin secretion. It prompts insulin secretion and contributes to the homeostasis of postprandial blood glucose levels.
K cells that secrete GIP are localized in the upper part of the small intestine, mainly in the duodenum and jejunum. Meanwhile, L cells that secrete GLP-1 are thought to be present in the lower part of the small intestine (Fig. 2)3). K cells and L cells sense nutrients that reach the intestinal tract and secrete GIP and GLP-1 on the cell basement membrane side, respectively. The rise of GLP-1 concentration in blood is observed immediately after dietary intake and lasts for 1 to 2 hours. Similarly, GIP increases immediately after dietary intake, and the increase in blood level continues for several hours. Carbohydrates are counted as a nutrient that promotes the secretion of incretins. When it comes to incretin secretion by carbohydrates, it is assumed that carbohydrates that were absorbed via sodium-glucose cotransporter 1 (SGLT1) cause the depolarization of cell membranes and facilitate the secretion of GLP-1 and GIP. In addition to carbohydrates, proteins and lipids are believed to promote the secretion of incretins.
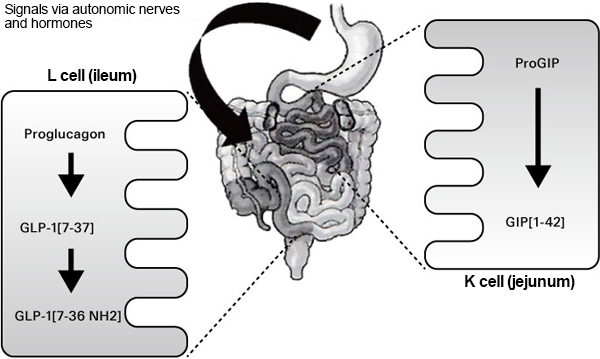
Fig. 2.Incretin synthetic secretion
Mitsuru Ohsugi (2010)3)
Exercise and glycometabolism
Glucose in blood is absorbed into cells via GLUT4. With healthy people, as the blood glucose level rises after meal ingestion, the pancreas secretes a corresponding amount of insulin. Then, insulin binds to the insulin receptor on a cell surface and the receptor issues signals to absorb glucose into the cells. Via this signal transduction, GLUT4 which is inside the cells, comes out onto the surface of the cells and absorbs the glucose in the blood into the cells. However, when insulin works poorly due to aging or diabetes, the signal via the insulin receptor is not transmitted well and GLUT4 does not come out onto the cell surface. If that is the case, the function to absorb glucose in blood into the cells decreases (insulin resistance). The amount of GLUT4 that appears on the surface of the cells is also increased by stimulating muscle contraction when exercising. In addition, since the amount of GLUT4 that appears on the surface of the cells increases with continued exercise, the amount of glucose to be absorbed into cells increases. As a result, homeostasis of the blood glucose level is maintained4).
The effects of exercise can be divided into acute effects and chronic effects. The acute effect indicates energy consumption. There is a hypothesis that AMP kinase detects an increase in AMP/ATP, which is associated with skeletal muscle contraction, and lowers the blood glucose level by increasing the amount of carbohydrates absorbed5). Since the mechanism of promoting glucose transport by exercise is different from that of the insulin response, the blood glucose levels of people who have insulin resistance are also lowered through exercise. The blood glucose level reaches its highest level in 30 minutes to 1 hour after a meal, and aerobic exercises during this time can restrain the rapid rise in postprandial blood glucose levels6).
Chronic effects include increasing insulin sensitivity. It has been suggested that this mechanism increases the amount of GLUT 4 protein, increases blood flow to motor muscles, decreases body fat, and increases insulin signaling protein7). Therefore, exercise restrains the elevation of blood glucose levels and is a factor for improving insulin resistance.
As for what types of exercise are considered effective, aerobic exercises such as walking, jogging and swimming, as well as muscle training, stretching, etc. are considered to be effective.
Diet and enzyme inhibitors
α-glucosidase inhibitor (GI) is a drug that limits glucose absorption from the upper part of the small intestine by inhibiting the actions of enzymes that decompose ingested carbohydrates into monosaccharides, which then restrains the rise in postprandial blood glucose levels. In Japan, three drugs are clinically used: acarbose (drug name: Glucobay), voglibose (drug name: Basen) and miglitol (drug name: Seibule). Acarbose and voglibose are poorly absorbed from the intestinal tract. However, a part of miglitol is absorbed in the small intestine and excreted from the kidneys in urine without being denatured. Acarbose inhibits not only the effects of α-glucosidase but also the effects of α-amylase (Fig. 3)2).
Foods also have the α-glucosidase inhibitory effect. Guava leaf polyphenol (maltase, sucrase and amylase inhibitory effect), wheat albumin (amylase inhibitory effect), L-arabinose (sucrase inhibitory effect), douchi (fermented black beans) extract (α-glucosidase inhibitory effect), etc. are used as food for specified health uses because the effect of restraining postprandial blood glucose level elevation can be expected.
Incretin is a hormone that is secreted by gastrointestinal secretory cells after meal ingestion and it enhances insulin secretion. However, among incretins, GLP-1 is rapidly decomposed and inactivated by dipeptidyl Peptidase-4 (DPP-4), whose presence can be observed throughout the body, and its half-life in blood is as short as 1 to 2 minutes. For this reason, incretin-related drugs have been drawing attention as new anti-diabetic drugs in recent years. In Japan, some medicinal drugs have been approved, such as liraglutide (drug name: Victoza) and exenatide (drug name: Byetta, Bydureon) as a DPP-4 resistant GLP-1 receptor drug, and sitagliptin (drug name: Januvia, Glactiv), vildagliptin (drug name: Equa) and alogliptin (drug name: Nesina) as a DPP-4 inhibitor3).
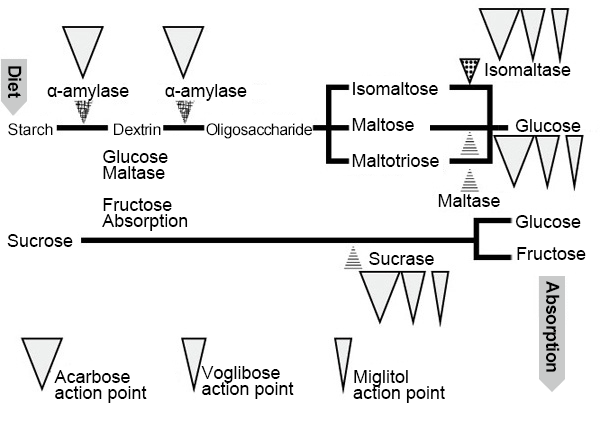
Fig. 3.α-glucosidase inhibitor action point
Kazuya Fujihara, et al. (2010) 2)
Diet that prevents postprandial blood glucose levels from easily increasing
In 1981, Jenkins et al. found that even if the amount of carbohydrates in the diet was the same, there were differences in the rate/degree of increase in blood glucose levels between each carbohydrates-based food (Fig. 4)8). They then used healthy non-diabetic volunteers to relatively compare each area under the glucose response curve (AUC) when they ingested 50 g of 62 types of food and the same amount of glucose. By doing so, the glycemic indexes (GI) of food was obtained and displayed. Since then, food GI has been used as one of the effective pieces of information in dietary guidance for diabetes and metabolic syndrome in clinical practice. On the other hand, GI shows the effects on blood glucose levels in terms of “quality” of carbohydrates when a certain amount of carbohydrates is ingested, but the effects on blood glucose levels differ depending on the “quantity” of ingested carbohydrates. Hence, Salmeron, et al. devised glycemic load (GL) as an index in consideration of both the quality and quantity of carbohydrates ingested during a single meal9). To obtain GL, the percentage of carbohydrates in food is indicated in percent and the value is calculated by multiplying the percentage by the food GI.
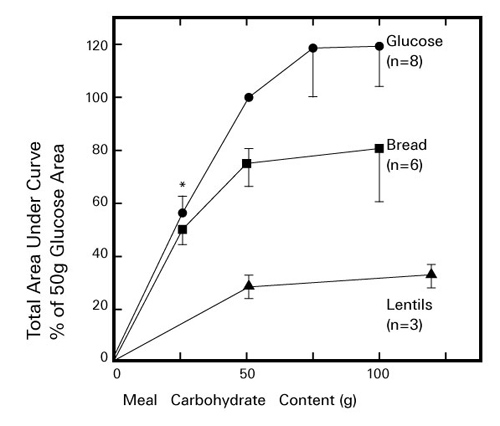 Fig. 4. Changes in blood glucose levels
Fig. 4. Changes in blood glucose levels
when healthy people ingest glucose, whole grain bread and lentils
Generally, GI values that are 70 or higher indicate the high GI foods, 56 to 69 indicate the middle GI foods and 55 or lower indicate the low GI foods. When ingesting low GI food, AUC becomes smaller compared to when ingesting high GI food. So, having low GI food may moderate the rise in postprandial blood glucose levels. The GI values vary depending on the amount of carbohydrates, the degree of purification, the amount of dietary fiber, the amount of lipids, the amount of protein, the degree of processing, etc. in foods. Additionally, it is an index that expresses the degree of increase in blood glucose levels as a relative value when 50 g of carbohydrates are ingested, with the value of the same amount of glucose set to 100 as a standard diet. However, it should be noted that the information shown as the GI values may be obtained from different types of food (rice, bread, etc.) and differing amounts of carbohydrates that are used as the standard diet. In Japan, the Japanese Association for the Study of Glycemic Index (JASGI) recommends the implementation of a protocol (unified method) which indicates 147 g of packaged rice (equivalent to 50 g of carbohydrates) as a standard diet10).
Also, adding dietary fiber to food may moderate the rise in postprandial blood glucose levels. Indigestible dextrin, a water-soluble dietary fiber, swells with water when ingested with carbohydrates and/or monosaccharides, slowing the rate of excretion from the stomach to the intestine and becoming a sticky gel in the small intestine to prevent food from spreading. When it becomes hard for degrading enzymes to come into contact with food, digestion and absorption of carbohydrates are delayed, thus, restraining the rise in postprandial blood glucose level. Foods containing indigestible dextrin are used in foods for specified health uses, which are expected to restrain the rise in postprandial blood glucose levels11).
On the other hand, the level of the rise in postprandial blood glucose levels changes depending on the order of food ingestion during a meal. It has been reported that when eating both 200 g of rice and 60 g of vegetables (salad), the increase in blood glucose levels and insulin secretion are moderated when eating vegetables before rice12). In response to the announcement of the results, health methods based on the eating order are gaining attention13). Additionally, it has been reported that postprandial hyperglycemia can be restrained by ingesting side dishes such as vegetables and/or hot spring (soft-boiled) eggs with udon noodles or rice (placed on top of udon noodles/rice) regardless of the eating order (Fig. 5)14).
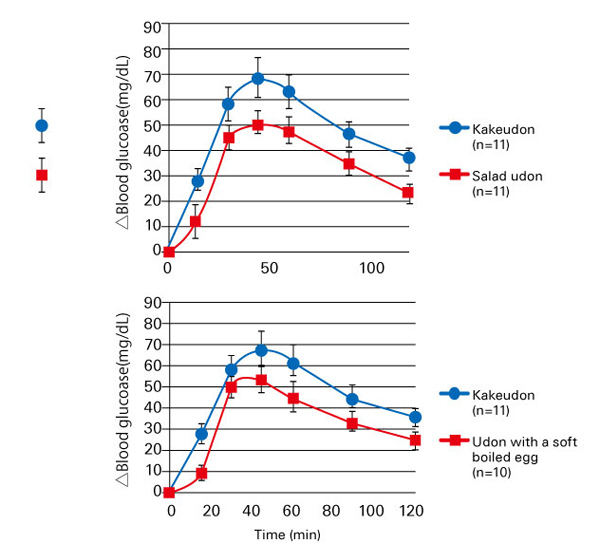 Fig. 5 Changes in postprandial blood glucose levels when ingesting udon noodles
Fig. 5 Changes in postprandial blood glucose levels when ingesting udon noodles
with salad/hot spring (soft-boiled) egg added to it
In recent years, low carb diets that reduce the dietary intake of carbohydrates themselves, which affect the rise in blood glucose levels, has become a hot topic. You should note that carbohydrates are an important nutrient and survey results show that extreme carbohydrate restrictions may increase the risk of death15).
References
-
- 永井竜児ら:基礎生化学 健康・疾病とのつながり. 2013, アイケイコーポレーション (東京).
- 藤原和哉ら:糖尿病の最新治療. 2010;2:6-15.
- 大杉 満:糖尿病の最新治療. 2010;2:21-26.
- 春日雅人:第116回医学会シンポジウム記録集 生活習慣と糖尿病の発症. 2000;13-18.
- Winder WW, et al.:Am J. Physiol. 1999;277:E1-E10.
- Derave W, et al.:Obesity. 2007;15:704-711.
- Chibalin AV, et al.:Pro Natl Acad Sci. 2000;97:38-43.
- Jenkins DJA, et al.:Am J Clin Nutr. 1981:362-366.
- Salmeron J, et al.:JAMA. 1997 ; 277:472-477.
- 日本Glycemic Index 研究会:http://www.gikenkyukai.com/protocol.html.
- 河合博成ら:健康・栄養食品研究. 2002;5:33-45.
- 金本郁男ら:糖尿病. 2010;53:96-101.
- 梶山静夫ら:なぜ、「食べる順番」が人をここまで健康にするのか:この食べ方で高血圧、糖尿病、高脂血症にならない! 2012, 三笠書房(東京).
- Matsushima M, et al.:Glycative Stress Research. 2014;1:53-59.
- Noto H, et al.:PLoS ONE 8(1):e55030. doi:10.1371 / journal.pone.0055030
Glycative stress and Anti-aging
- What is glycative stress?
- Glycative stress biomarker measurement method (1) Measurement of blood glucose, glycated protein and glycation reaction intermediate
- Glycative stress biomarker measurement method (2)AGEs measurement
- Glycative stress biomarker measurement method (3) Evaluation of anti-glycative effects
- Glycative Stress and AGEs Receptors
- What is kidney disease?
- Glycative Stress and Skin Aging
- Glycative stress and arteriosclerotic disease
- Glycative stress and schizophrenia
- Glycative stress and liver disease
- Glycative stress and infertility
- Glycative stress and Alzheimer’s disease
- Glycative stress countermeasures (1) Blood glucose control
- Glycative stress countermeasures (2) Inhibition of glycation reaction
- Measures against glycative stress (3) Degradation and excretion of AGEs
- Measures against glycative stress (4) AGEs contained in food
- Issues and prospects of glycative stress countermeasures
- Issues and prospects of glycative stress countermeasures