Glycative stress and Anti-aging
Glycative stress and arteriosclerotic disease
Glycative stress and arteriosclerotic disease
Classification and definition of arteriosclerosis
Arteriosclerotic disease mainly indicates cardiovascular disease and cerebrovascular disease. In recent years, it has been recognized as a multiple vessel disease that includes peripheral arterial disease 1).
Arteriosclerosis is a general term for arterial lesion that indicates the thickening, hardening and rebuilding of the arterial wall, and is classified into atherosclerosis, arteriolosclerosis and Monckeberg’s medial calcific sclerosis. Atherosclerosis is an arterial lesion that is deeply related to the onset of lifestyle diseases and occurs in a relatively large elastic artery. When histologically considering atherosclerosis, it is a lesion that lipid-rich necrotic cellular debris, which is called atheroma, accumulates in vascular intima, and fibrocellular capsules cover its surface2). Atherosclerosis is defined as “a lesion of the accumulation of lipid, acid mucopolysaccharide, blood-derived materials, fiber and calcium deposition due to changes mainly in the intima.” by the World Health Organization (WHO).
Atherosclerosis and AGEs
The forming of atherosclerosis is affected by multiple mechanisms. The initial lesion from atherosclerosis is called a fatty streak. In the fatty streak, blood-derived lipids are deposited into the vascular intima of blood vessels that are thickened via the cellular/fibrous mechanisms, and accumulation of macrophage-derived foam cells and T lymphocyte infiltration occur. This lesion is induced when lipoprotein, especially LDL (low-density lipoprotein), passes through endothelial cells from blood, binds to proteoglycan and accumulates in intima 2).
Glycated LDL is not detected by a LDL receptor and accumulates in endothelial cells. When glycated LDL is aging, it combines with an AGEs receptor (RAGE) that is expressed in vascular cells such as endothelial cells or smooth muscle cells, and increases the expression of proinflammatory cytokine or cell adhesion factors via production of reactive oxygen species (ROS), activation of protein kinase C (PKC) and activation of mitogen-activated protein kinase (MAP kinase). As a result, smooth muscle cells migrate/proliferate, endothelial cell dysfunction, and the acceleration of oxidative stress occur. ROS that was produced via oxidative stress, oxidizes vascular wall cells as well as glycated LDL. When oxidation of glycated LDL progresses, lipid hydroperoxide (one of the types of lipid peroxide) accumulates, and produces malondialdehyde (MDA), a secondary product. In response to the glycation of LDL, hexitollysine (HL) is produced. On the other hand, with presence of ROS, CML is produced from LDL. Oxidized, glycated or glycatively/oxidatively modified LDL accumulates inside the intima (Fig. 1)4). The progression of connectivity to collagen affects the accumulation of oxidized LDL5).
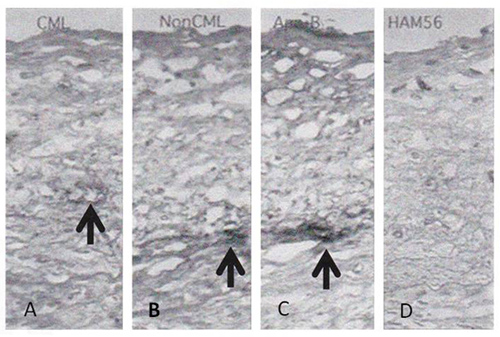
Fig. 1 AGEs during fibrocellular intimal thickening
A: CML antibody staining,
B: anti AGEs (non-CML) antibody staining,
C: anti ApoB antibody staining,
D: anti-macrophage (HAM56) antibody staining
The dark colored areas that the arrows point to indicate accumulation.
Adapted from Noriyuki Sakata (2012)4)
Along with produced CML and oxidized phosphatidylcholine (OxPC), which is a lipid peroxide, modified LDL that has accumulated in intima is combined with macrophages that have infiltrated the intima and become foam cells 6). On the other hand, ApoB (a type of apolipoprotein) and pyrraline (a type of AGE), which are indexes of arteriosclerotic diseases, are not combined with macrophage. Instead, they are distributed over the extracellular matrix of intema (Fig. 2) 6).
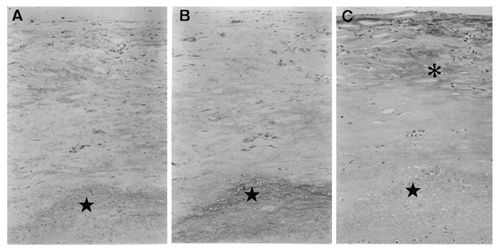
Fig. 2 AGEs accumulated in fatty streak
A woman who died at age 70 from malignant lymphoma
A: anti malondialdehyde (MDA) antibody staining,
B: anti CML antibody staining
C: anti pyrraline antibody staining,
★: atheroma area, *: pyrraline accumulation area
Adapted from Sakata N, et al (2001)6)
And, proinflammatory cytokine such as TNF-α, TNF-β and IL, and/or proliferation factors are emitted from macrophage. Therefore, smooth muscle cells transform and promote migration/proliferation and collagen production. In addition, since proteolytic enzymes such as matrix metalloproteinase are also secreted, intimal tissue destruction and restoration are repeated. As a result, atherosclerosis is formed in intima. The rupture, malignant transformation and hemorrhaging of capsula fibrosa that cover atheroma may cause acute coronary syndrome or cerebral infarction due to formed blood clots.
Arteriosteogenesis and AGEs
Calcification is observed in atheroma with advanced atherosclerosis, which is thought to be the denaturation of arterial walls and the final stage of the necrosis processes. When smooth muscle cells or foam cells around an atherosclerosis area cause apoptosis, cytoclasis follows it and membranous vesicles are released in the extracellular matrix, which produces hydoroxyapatite. Mineralization here will cause amorphous calcification.
Diabetes promotes vascular calcification. With diabetes, glycation progresses, which causes the aging of collagen in arterial walls. Then, the molecular structure of collagen with AGEs changes, making calcification easy to occur 2). Therefore, granular calcification, which is the initial lesion of Monckeberg’s medial calcific sclerosis, can be seen in the arterial medium of diabetics. CML is present in calcified media. It is indicated that CML is also formed in arterial collagen 7).
With patients with renal failure, calcification can be seen along the elastic fiber of an aortic elastic artery, etc. Vascular calcification with patients with renal failure is affected by a metabolic disorder (calcium or phosphorus), as well as increasing AGEs in serum (e.g. peptide, pentosidine and CML) and dicarbonyl compounds (glycation reaction intermediate) 8-9). With renal failure, AGEs and glycation reaction intermediates accumulate inside the body while accelerating the vicious cycle of AGEs. As a result, angiosclerosis that includes atherosclerosis advances, leading to the progression of calcification.
Vascular calcification leads to artery hypofunction, which not only causes increased pulse pressure, cardiac stress and peripheral circulatory failure, but also affects the destabilization of atherosclerosis. Therefore, controlling arteriosteogenesis through AGEs is significant for the prevention or improvement of cardiovascular complications in patients with renal failure or diabetes.
References
-
- 寺本民生:栄養学雑誌. 2013 ; 71 : 3-13.
- 坂田則行ら:動脈硬化と糖化, 糖化による疾患と抗糖化食品・素材. 2011 : 66-76, シーエムシー出版.
- Nakashima Y, et al. : Arterioscler Thromb Vasc Biol. 2007 ; 27 : 1159-1165.
- 坂田則行:アンチエイジング医学. 2012 ; 8 : 42-48
- Jimi S, et al. : Atherosclerosis. 1994 ; 107 : 109-116.
- Sakata N, et al. : Cardiovasc Res. 2001 ; 49 : 466-475.
- Sakata N, et al. : J Vasc Res. 2003 ; 40 : 567-574.
- Sakata N, et al. : Nephrol Dial Transplant. 2003 ; 18 : 1601-1609.
- Miyata T, et al. : J Am Soc Nephrol. 1998 ; 9 : 2349-2356.
Glycative stress and Anti-aging
- What is glycative stress?
- Glycative stress biomarker measurement method (1) Measurement of blood glucose, glycated protein and glycation reaction intermediate
- Glycative stress biomarker measurement method (2)AGEs measurement
- Glycative stress biomarker measurement method (3) Evaluation of anti-glycative effects
- Glycative Stress and AGEs Receptors
- What is kidney disease?
- Glycative Stress and Skin Aging
- Glycative stress and arteriosclerotic disease
- Glycative stress and schizophrenia
- Glycative stress and liver disease
- Glycative stress and infertility
- Glycative stress and Alzheimer’s disease
- Glycative stress countermeasures (1) Blood glucose control
- Glycative stress countermeasures (2) Inhibition of glycation reaction
- Measures against glycative stress (3) Degradation and excretion of AGEs
- Measures against glycative stress (4) AGEs contained in food
- Issues and prospects of glycative stress countermeasures