Glycative stress and Anti-aging
Glycative stress and liver disease
Glycative stress and liver disease
What is liver disease?
The liver, which is an organ that takes on about 25% of basal metabolism, metabolizes carbohydrates, lipids, proteins and vitamins, detoxifies harmful substances, and secretes bile. In addition, with its excellent regenerative ability, the liver is a regenerative organ that can be regenerated even if its liver cells are destroyed. On the other hand, liver diseases include a number of diseases for different pathological conditions such as hepatitis, cirrhosis, and fatty liver disease. The liver is also called a “silent organ” because it does not show any subjective symptoms of a liver disease.
Depending on the condition, hepatitis is mainly classified into acute hepatitis and chronic hepatitis (where inflammation continues for 6 months or more). It is also classified by its cause into viral type (type A, B, C and others), alcoholic, drug-induced, autoimmune hepatitis, and others 1).
Hepatitis A is one of the infections of the digestive tract caused by the hepatitis A virus (hepatitis A virus: HAV). A major cause of infection is consumption of contaminated shellfish and other foods, and is commonly observed in regions with poor sanitation. When infected as a child, it usually results in a subclinical infection, but it often develops when infected as an adult. Hepatitis B, which is caused by the hepatitis B virus (HBV), can be transitory or persistent. Most persistent cases are due to mother-to-child transmission, which in many cases results in acute hepatitis and does not become chronic. Hepatitis C, which is caused by the hepatitis C virus (HCV), develops regardless of season, age, or sex. Hepatitis C accounts for the majority of cases of hepatitis that are caused by blood transfusion, and it has a high probability of becoming chronic.
Until recently, most of the causes of liver cancer, which is one of the liver diseases, were viral liver diseases such as hepatitis C. However, in recent years, non-viral cases have been increasing as a cause of liver cancer, many of which are considered to be caused by fatty liver.
A fatty liver is caused by obesity, excessive energy due to too much intake of alcohol and sugar, diabetes, lack of exercise, etc. Consumption of alcohol equivalent to about 5 glasses (about 900 ml) of Japanese sake per day for 1 consecutive week can lead to a fatty liver even in a healthy person 2-4). Ten to twenty percent of people with alcoholic fatty liver who continue consuming an excessive amount of alcohol can develop alcoholic liver disease (ALD). ALD progresses from alcoholic fatty liver to alcoholic hepatitis, and then to alcoholic cirrhosis.
On the other hand, fatty liver includes clinical conditions called nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). NASH occurs in the context of fatty liver associated with overeating, lack of exercise, obesity, diabetes, dyslipidemia, etc. despite no history of drinking alcohol. Unless any treatment or intervention takes place, 5 to 20% of patients with NFLD will develop cirrhosis or liver cancer in 5-10 years 5). NASH and ALD show similarities in liver histology, both of whose pathogenic mechanisms have been studied.
Liver disease and AGEs
Among various types of AGEs generated in vivo, it has been reported that glyceraldehyde-derived AGEs (Glycer-AGEs) and acetaldehyde-derived AGEs (AA-AGEs) are involved in liver diseases 6). Up until now, it was thought that that AGEs are produced in vivo mainly from glucose and proteins. However, it has been confirmed that they are also produced from metabolic intermediates and degradation products of glucose, glycation reaction intermediates, fructose, and so forth (Fig. 1) 7). Furthermore, compared to glucose-derived AGEs (Glc-AGEs) and fructose-derived AGEs (Fru-AGEs), other AGEs are formed faster. For example, when bovine serum albumin (BSA) is used as protein, AGE can be formed easily in the following order: Glycer-AGEs, glycolaldehyde-derived AGEs (Glycol-AGEs), methylglyoxal-derived AGEs (MGO-AGEs), glyoxal-derived AGEs (GO-AGEs), 3-deoxyglucosone-derived AGEs (3-DG-AGEs), Fru-AGEs and Glc-AGEs.
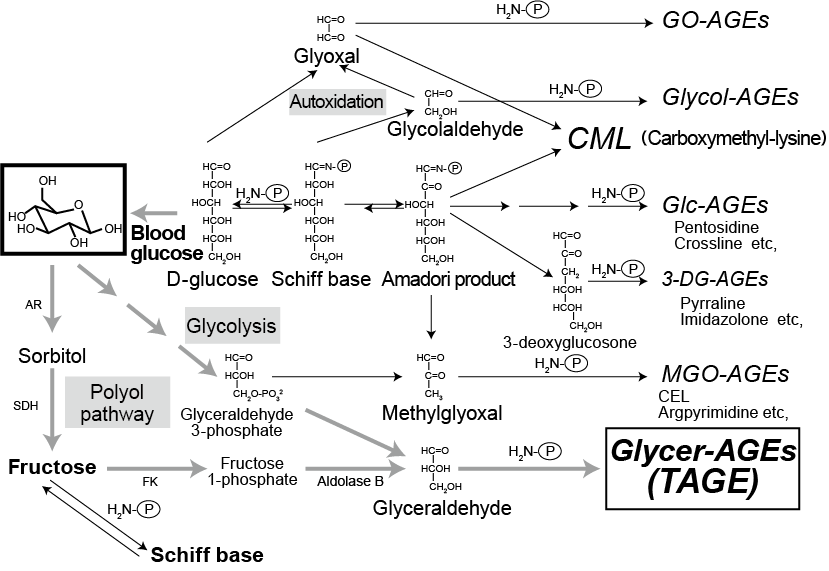
Fig. 1 In vivo pathways for forming AGEs
Glc-AGEs: glucose-derived AGEs; Fru-AGEs: fructose-derived AGEs; Glycer-AGEs: glyceraldehyde-derived AGEs;
Glycol-AGEs: glycolaldehyde-derived AGEs; MGO-AGEs: methylglyoxal (MGO)-derived AGEs;
GO-AGEs: glyoxal (GO)-derived AGEs; 3-DG-AGEs: 3-deoxyglucosone (3-DG)-derived AGEs;
CML: N-(carboxymethyl)lysine; AR: aldose reductase; SDH: sorbitol dehydrogenase; FK: fructokinase, H2N-P: free amino group in protein
Masayoshi Takeuchi (2012) 3)
In particular, Glycer-AGEs are strongly involved in the onset and progression of diabetic vascular complications via receptors for AGEs (RAGE) 8-9). When Glycer-AGEs are added to a hepatocyte culture system, one of the inflammatory markers, C reactive protein (CRP), will increase. Similarly, adding Glycer-AGEs to a culture system of hepatic stellate cells, which are associated with hepatocyte fibrosis, will induce oxidative stress via RAGE and cause inflammation 10).
Furthermore, the following matters have been also confirmed: Glycer-AGEs are accumulated in the liver of a NASH patient who does not have impaired glucose tolerance 11); when glyceraldehyde is added to the cell culture system of Hep3B, which is a human liver cancer cell, the increase in Glycer-AGEs in the hepatocytes causes cell death; and if heat shock cognate 70 (Hsc70), one of the molecular chaperones, turns into Glycer-AGEs, the chaperone activity decreases and hepatocellular injury may develop thorough protein dysfunction. (Fig. 2) 7, 12).
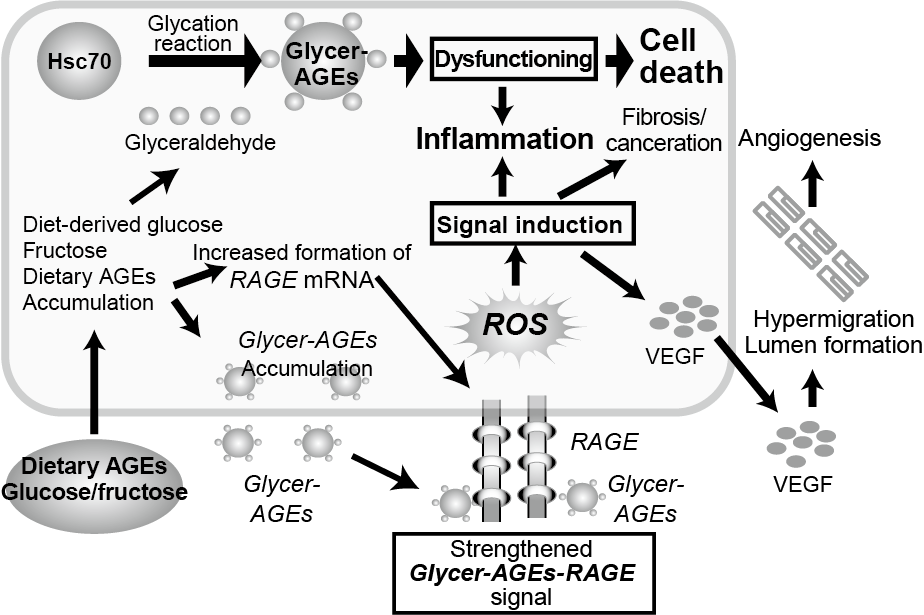
Fig. 2 Effects of Glycer-AGEs on hepatocytes in the onset/development of NASH
HSC-70: heat shock cognate 70; Glycer-AGEs: glyceraldehyde-derived AGEs;
RAGE: receptor for AGEs; ROS: reactive oxygen species; VEGF: vascular endothelial growth factor
Masayoshi Takeuchi (2012) 3)
On the other hand, a part of acetaldehyde, which is a metabolite of alcohol, binds to protein to form AA-AGEs (Fig. 3). AA-AGEs have strong effects on hepatocellular injuries. They are mainly formed in hepatocytes around the central veins, thus they are deeply involved in the onset and progression of ALD 13). It has been reported that AA-AGEs are present in the blood and brain of alcohol dependent patients 14) and the accumulation of AA-AGEs was observed in hepatocytes around the central veins in a rat model of chronic alcohol consumption.These suggest that even after acetaldehyde is eliminated from the blood by alcohol metabolism, AA-AGEs may pass on toxic effects to nerve cells and hepatocytes, thus they are involved in the onset and progression of alcoholic liver disease.
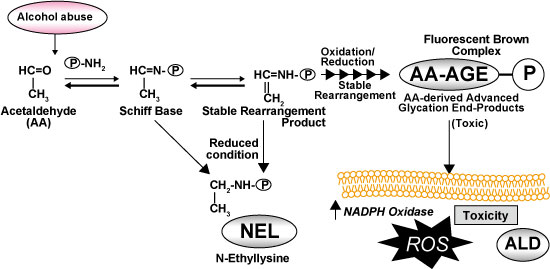
Fig. 3 Formation and effects of acetaldehyde-derived AGEs due to intake of alcohol
Hayashi N, et al .(2013)14)
Effects of dietary AGEs on the liver
Various AGEs are formed in food during the heating and processing stages of food. Many commercial foods contain mainly Glc-AGEs and Fru-AGEs. According to the the study where beverages containing a high level of Glc-AGEs were orally administered to normal rats to examine the RAGE expression system caused by Glycer-AGEs, the elevated expression of the vascular endothelial growth factor (VEGF) gene, an accumulation of Glycer-AGEs and Glc-AGEs in the liver were observed 15).
Also, the formation of Glycer-AGE being associated with the postprandial blood glucose fluctuations was confirmed in a restricted feeding experiment system using type 2 diabetes model rats 16). It also demonstrated that Glycer-AGE levels in the blood significantly decreased when an α-glucosidase inhibitor was administered to patients with type 2 diabetes 17). Moreover, a study reported that sugar intake from soft drinks is 5 times higher in patients with NAFLD than in healthy people, indicating that the excessive intake of sugar and high-fructose corn syrup, which is added to beverages as a sweetener, is associated with the onset and progression of NAFLD. These results suggest that dietary AGEs and postprandial hyperglycemia may elevate Glycer-AGEs levels in the blood, enhance the interaction with the RAGE expression system via Glycer-AGEs, and cause liver diseases 18).
References
-
- 原田 尚 : 日内会誌. 1991 ; 80 : 1563-1567.
- Bellentani S, et al. : Gut. 1997 ; 41: 845–850.
- Becker U, et al. : HEPATOLOGY. 1996 ; 23 : 1025-1029.
- 石井裕正:日本内科学会雑誌. 2003 ; 92 : 45-49.
- 日本肝臓学会:NASH・NAFLDの診療ガイド2010
- 竹内正義:アンチエイジング医学. 2013 ; 8:55-61.
- 竹内正義:金医大誌. 2012 ; 37:141-161.
- 竹内正義ら:生体の科学. 2007 ; 58:502-511.
- Takeuchi M, et al. : Curr Drug Targets. 2010 ; 11 : 468-1482.
- Iwamoto K, et al. : J Gastroenterol. 2008 ; 43 : 298-304.
- Hyogo H, et al. : J Gastroenterol Hepatol. 2007 ; 22 : 1112-1119.
- Takino J, et al. : J Gastroenterol Hepatol. 2010 ; 45 : 646-655.
- Takeuchi M, et al. : J Neuropathol Exp Neuro. 2003 ; 62 : 486-496.
- Hayashi N, et al. : PLoS ONE 8(7) : e70034.
- Patel R, et al. : PLoS ONE 7(4): e35143.
- Kitahara Y. et al. : Clin Exp Med. 2008 ; 8 : 175-177.
- Tsunosue M, et al. : Clin Exp Med. 2010; 10 : 139-141.
- Sato T, et al. : Eur J Nutr. 2009 ; 48 : 6-11.
Glycative stress and Anti-aging
- What is glycative stress?
- Glycative stress biomarker measurement method (1) Measurement of blood glucose, glycated protein and glycation reaction intermediate
- Glycative stress biomarker measurement method (2)AGEs measurement
- Glycative stress biomarker measurement method (3) Evaluation of anti-glycative effects
- Glycative Stress and AGEs Receptors
- What is kidney disease?
- Glycative Stress and Skin Aging
- Glycative stress and arteriosclerotic disease
- Glycative stress and schizophrenia
- Glycative stress and liver disease
- Glycative stress and infertility
- Glycative stress and Alzheimer’s disease
- Glycative stress countermeasures (1) Blood glucose control
- Glycative stress countermeasures (2) Inhibition of glycation reaction
- Measures against glycative stress (3) Degradation and excretion of AGEs
- Measures against glycative stress (4) AGEs contained in food
- Issues and prospects of glycative stress countermeasures